Abstract
Sickle cell disease (SCD) is a severe hemoglobinopathy due to abnormal hemoglobin S (HbS). Although red blood cell dysfunction is at the core of the SCD pathophysiology, several studies have highlighted the important role of inflammatory cells like neutrophils. One of the most serious complications of SCD is cerebral vasculopathy (CV), due to the occlusion of one or more intracranial or cervical arteries. In 1998, the STOP study demonstrated that monthly blood transfusions could reduce the risk of stroke by 90% in children with CV. However, there is large heterogeneity in the evolution of CV under chronic transfusion, sometimes requiring exchange transfusion (ET) program for years without succeeding in healing the CV.
The aim of the study is to investigate the impact of long-term transfusion program on neutrophil dysfunction, in order to understand if persistent inflammation could contribute to the non-healing of CV despite HbS permanently below 40%. In SCD children undergoing ET program for at least 1 year, we analysed i)the phenotype of neutrophils with 8 markers of activation/adhesion/ageing, ii)the plasmatic levels of elastase, witnessing the NETose activity of neutrophils, and iii)the ex-vivo adhesion of neutrophils on activated endothelial cells.
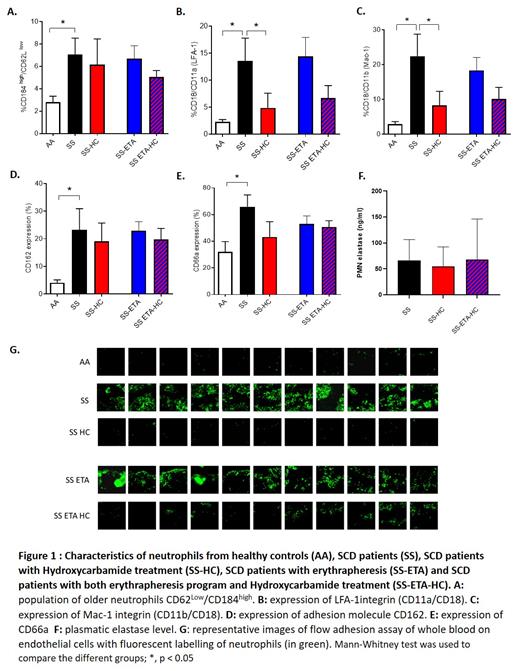
One hundred and two SCD children with an ET transfusion program for at least 6 months because of CV were included in the study. ET session, carried out every 5 weeks and most of the time by erythrapheresis, reached their biological objectives with a mean HbS rate after ET session of 14.1%, and 35.4% before the next ET session, which means that these patients globally live at an average HbS level of 24% for at least 1 year. We managed to limit iron overload with a mean ferritinemia of 207 µg/L in the whole cohort. Despite these satisfactory results in terms of HbS reduction, the efficiency in curing the CV was modest in accordance with the previously described efficiency of ET program in SCD children: after a mean ET program duration of 4.4 years only 22% of them had an improvement of their CV since the beginning of the ET program, while 60% of them had a stagnation of their CV, and 18% of them worsened their vascular lesions. Considering inflammatory parameters, the patients had persistence of high leukocytosis and high neutrophils count (respective mean of 9810 G/L and 5742 G/L), significantly not different of neutrophils count before inclusion in the ET program. In a random subgroup of 20 patients, we analysed neutrophils phenotype, NETose and endothelial adhesion and compared them to healthy controls and SCD children without ET, treated or not with Hydroxyurea (HU). Overall, we observed as expected an activated, aged and adherent profile of neutrophils from untreated SCD children compared to healthy controls, characterized by an overexpression of CD18/CD11b (p=0,03), CD18/CD11a (p=0,02), CD162 (p=0,01), CD66a (p=0,01) and the ageing markers CD184 high/CD62Llow (p=0,04) as well as a higher plasmatic level of elastase (p=0. 01) and higher adhesion of neutrophils to endothelial cells. All these parameters were alleviated in SCD patients treated with HU. In SCD patient undergoing ET program, we found a similar profile of activated neutrophils to that of untreated SCD patients with a similar expression of activation molecules, high level of elastase and the same increase of neutrophils adhesion to endothelial cells compared to controls, witnessing a persistence of chronic inflammation despites years of ET.
Overall, our study highlights that the replacement of sickle red blood cells, even for years, is not sufficient to reverse the deleterious inflammatory phenotype of neutrophils. Given the major role of inflammation in endothelial dysfunction, these could contribute to the persistence of CV in a majority of patients despite efficient ET programs. This raises the question of systematically combining ET program with anti-inflammatory treatment such as HU or P-selectin inhibitors in children with CV.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal